Human Studies
First Observational Study 2010
During 2010 an observational study was carried out including 30 patients suffering osteoarthritis (17 women and 13 men), to whom a daily dosage of 8 g Jointsol® was supplied.
Average age of participants was 60.6 years and BMI was 29.7. All participants showed a degree of osteoarthritis between 2.2 and 3.8. The length of the study was 12 weeks evaluating several parameters among them the degree of mobility, pain and quality of life. Results
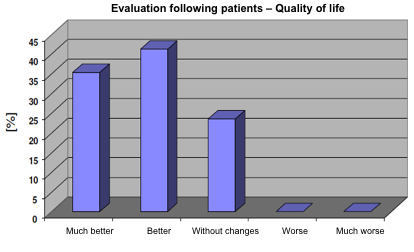
At the end of the study 75% of patients evaluated the treatment with Jointsol® as effective due to the improvement in their quality of life. 40% considered the treatment as much better and 35% as better when compared to the start of the study. Only 25% of patients considered that the treatment did not change their quality of life and none of the patients get worse with the treatment with Jointsol® (fig. 5).
An evaluation of joint paint evolution under the treatment was done at weeks 3, 6 and 12. This evaluation considered the periods of activity and the resting time, as well as the degree of mobility following the evaluation of joint flexibility (fig. 6).
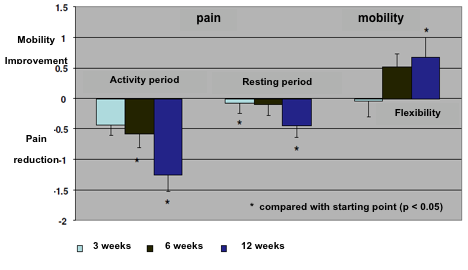
With regard to the joint pain evaluation it was measured in the three mentioned periods. During the activity period a reduction of pain symptoms was obtained after the three weeks of treatment. Such reduction increased trhoughout the study and was statistically significant at the end of the study. This pain reduction was also observed during the resting period though this reduction was not as great as that obtained in the activity period. Nevertheless, taking into account all the data available researchers concluded that the reduction in pain was significant after 12 weeks of treatment.
As joint pain is clearly higher during activity periods than resting periods, the results showed that Jointsol® is really effective when used actively to improve mobility reducing pain at the same time.
Second Observational Study 2011
At the end of 2010 and beginning of 2011 a second observacional study with Jointsol® was carried out. This study differed from the first one with regard to the regime dosage that was 1 or 2 sachets daily (containing 8 g of Jointsol® each).
With this dose regimen a 12-week non-interventional uncontrolled observational multicenter study in patients (n=108) with osteoarthritis (clinically and radiologically verified) was done.
The study population was older than 50 years and characterized by uni- or bilateral gonarthrosis (OA level 1 or 2) of the knee joints.
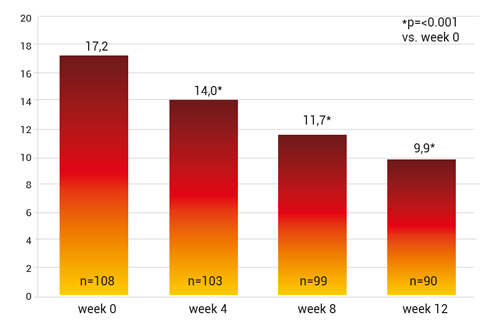
Regarding the primary study objective the WOMAC-Score lowered the pain scale by 6.7 points, p<0.001 vs. starting score and the standard medical pain therapy was reduced by 15.5% (fig. 7).
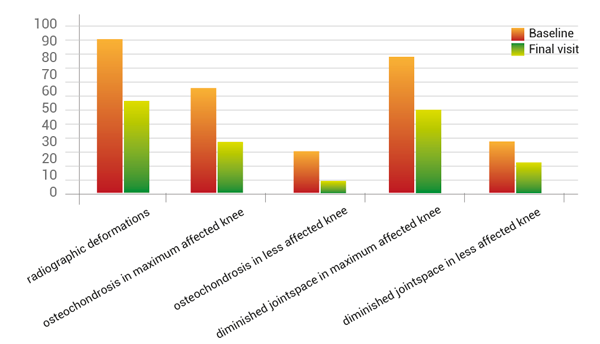
Secondary parameters showed improvements of diminished joint space (-32.7%), osteochondrosis (-33.8%), goniometric rise of flexibility (3°), as well the reduction of morning stiffness of about 20% measured from starting to endpoint (fig. 8).
The results show first steady improvements after 4 weeks and most significant improvements after 8 weeks. The compliance for regular daily intake was >90%, whereof 85% of the patients took 1 sachet per day. No significant difference in efficacy could be detected in comparison to the daily intake of 2 sachets.
This human study confirmed the former in vitro data. It can be summarized that the new rose hips extract preparation shortens the time of observed effects to 4-8 weeks compared to pure hydrolyzed collagen, which needs from 12 to 24 weeks to see positive effects, or compared to rose hip powder alone, which needs 12 weeks to see pain reduction in affected joints.
These studies revealed that half of the single dosage is effective in pain reduction and improvement of mobility, all supported by radiological evidence.
Conclusion
Pre-clinical studies which have been carried out with Jointsol®, a special patented combination of rose hip and collagen, showed a positive effect on inflammation degree and regeneration in joint tissue, greater than the one that can be obtained by the use of these substances separately. This demonstrates that the mixture has a synergic effect in the treatment of inflammatory and degenerative processes of joints.
The collagen matrix acts as a structural restorative of the fundamental substance components stimulating the anabolic processes and the mechanisms, which activate the regenerative action of chondrocytes and osteoblasts.
The rose hip has an inhibitory action in releasing pro-inflammatory metabolites (interleukin and tumoral necrosis factor) and reduces the stimulation of inflammatory cells. This process diminishes the degradation of collagen and glycoproteins of the extracellular matrix by the action of metalloproteinases, producing an anti-inflammatory effect on joints.
The regenerative effectiveness of the hydrolyzed collagen is developed by the existence of a special aqueous extract obtained by rose hip skins. This combination creates a commercial product, Jointsol®, which allows a remarkable reduction in the quantity of hydrolyzed collagen which could be provided as a therapeutic dose to treat the inflammatory and degenerative processes of the musculoskeletal system.
The two observacional studies carried out in patients suffering from osteoarthritis have shown that the treatment with Jointsol® improves pain symptoms of joints at 3 weeks from starting the intake. This improvement is observed during the activity phase, which is the most painful and it is followed by a significant reduction of joint pain at week 4. The pain reduction is associated with an increase in joint mobility, which is totally evident after 12 weeks of treatment. These results confirm the efficacy of Jointsol® for the treatment and regeneration of joints as well as preventing joint degeneration.
Jointsol® clearly shows an anti-inflammatory action in the osteoarthritis process. This positive reduction of inflammation allows the chondrocytes to regenerate the damaged cartilage of the joint. This fact has been demonstrated in in vitro studies.
The combination of the stimulating effect of hydrolyzed collagen on cartilage bone growth together with the anti-inflammatory action of a special hip rose extract makes Jointsol® a third generation product for the treatment of osteoarthritis and related illnesses.
This special blend opens new possibilities for the treatment of bone-joint illnesses as well as the prevention of joint cartilage degeneration in people who submit their joints to special efforts such as sportsman.
Observational Study Poster

Continue

